PolymerExpert designs custom specialty polymers for its customers. Whether in the medical, cosmetic or industrial fields, our productions are subject to batch release to ensure product conformity and quality. This requirement allows us to guarantee to the customer the conformity of the product to the required specifications and allows a traceability. Quentin Monange, operational quality expert explains the process.
Over the years, PolymerExpert has seen its activity as a polymer research and development laboratory evolve. Indeed, by moving from a strategy of off-the-shelf specialty polymers to a strategy of custom production with an increase in scale, the company had to implement a strict process to ensure the quality of its productions.
Historically focused on the medical sector, PolymerExpert has diversified to become a major player in the cosmetics and industrial sectors. The possibility of having several production lines allows the company to respond to numerous requests from industrialists in all sectors. At the end of the production process, all the productions are subjected to the same requirement: the release of the batch.
This step of batch release is essential to guarantee the conformity of the synthesized materials to the customer’s standards, and thus guarantee his satisfaction.
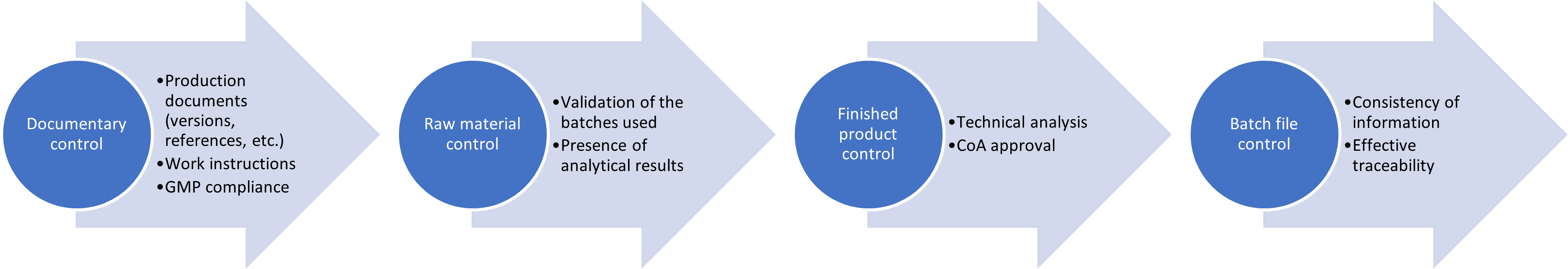
This strict process, which is based on both our internal requirements and normative requirements, is described as follows
- Documentary control of production documents: the versions and references of the working documents used, the presence of all the required information, the rules of drafting in accordance with Good Manufacturing Practices (GMP)
- Control of the conformity of purchased raw materials: although a release process dedicated to raw materials already takes place before production, a new control is carried out afterwards to ensure the conformity of the product to our internal specifications.
- Control of the conformity of the finished product: throughout the production process, analyses, carried out by our experts in analysis and with the help of our state-of-the-art analytical equipment, are carried out on the product to guarantee its conformity to the desired specifications. These results are summarized in a Certificate of Analysis (CoA).
- Control of the final batch file: this file gathers all the evidence of the above-mentioned controls, allows us to control the coherence of all the information that can be found there and to establish the internal traceability of the products. This is the last step at the end of which a batch release certificate is issued.


