At the beginning of the film The Graduate, when Mr McGuire talking to Benjamin, trying to give the young graduate a one-word advice for his future career: “Plastic!”, and Benjamin was stunned. It is true that “plastic” is a fast-developing industry and a good deal in 50-60s, but it also means “cheap, ugly, unreal” at that time. Today, plastic, almost a synonym of polymer, presents in nearly everything in our daily life thanks to its versatile properties. However, the wide application of polymers has also brought a lot of problems such as marine litter, microplastic pollutions etc. In this case, development of alternative, biodegradable materials became urgent and important. Polysaccharide, a biopolymer which is composed of covalently linked sugar molecules, is the largest resource of polymer from the nature. Thanks to its availability, biocompatibility and biodegradability, polysaccharide could replace synthetic polymers in many domains: one best example is cosmetics. At PolymerExpert, we have already a product portfolio for cosmetic, especially our recent products: EstoGel® Green, which is a 100% biobased oil phase gelling agent. For enriching our portfolio of cosmetic products, polysaccharide could be a very promising resource of raw material.
Cellulose and its derivatives
Cellulose is the most abundant polymer resource which comes from nature, it is a beta-D-1,4 linked glucose unit. Cellulose is the main ingredients of plant cell wall, it might originated from wood, cotton linters and bacteria. Bacterial cellulose is already widely used in cosmetic thanks to its high capacity of water retention and skin permeability (Biotechnology Reports, 2020, 27, e00502).
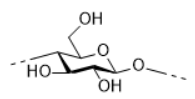
Cellulose nanocrystal (CNC) or Cellulose nanofiber (CNF) could be obtained by acid, enzymatic hydrolysis or oxidation of cellulose, which are applied to remove the amorphous domains of cellulose to yield rodlike cellulose particle with a size of 5-20 nm in diameter and 100 nm in length. Unlike cellulose, CNC could be well dispersed in water to form a stable emulsion, or hydrogel, or even aerogel and maintained the properties of cellulose such as hydrophilicity, biocompatibility (Angew. Chem. Int. Ed. 2011, 50, 5438; Chem. Mater. 2017, 29, 4609).
Cellulose could be functionalized to have more interesting properties, such as alkyl cellulose (methyl, ethyl, propyl), carboxymethylcellulose, hydroxyalkyl cellulose (methyl, ethyl, propyl, methyl/propyl,…), etc., which already have a wide application in cosmetics as surfactant, stabilizer, thickening agent.
Chitosan
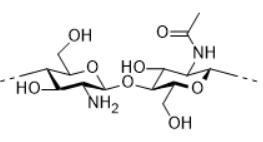
Chitosan is obtained from partially deacetylated chitin which is the main component of exoskeleton of crustacea animals, insects, or mushrooms. The amine group of chitosan could be protonated in acidic conditions, its pH responsive water solubility and bioactivity allow a lot of applications in pharmaceutic and cosmetic. Chitosan could also be derivatized to improve their properties thanks to the amine groups.
Algae polysaccharides
Seaweed is another rich resource of polysaccharides. Alginate, carrageenan are frequently used algae polysaccharides in cosmetics.
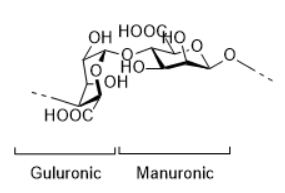
Alginic acid is gained from brown algae. The polysaccharide is composed of varying ratios of mannuronic acid and guluronic acid. Alginic acid and its calcium salt is insoluble in water but can swell in it, so it could be used for thickening, or water retention film, and could bind heavy metal ions which are involved in radical formation. However, the sodium and potassium salt of alginic acid, namely sodium or potassium alginate, are both water-soluble.
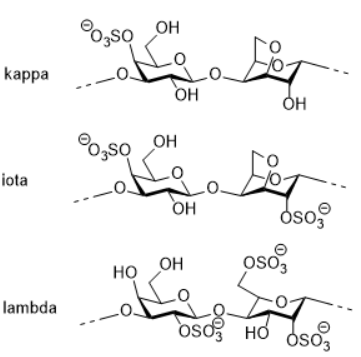
Carrageenan is extracted from red algae, the composition of carrageenan’s repeating units are 1,4 linked galactose and 3,6-anhydro galactose, with different content of sulfates. Based on sulfates content, there are kappa (low), iota (medium) and lambda (high) carrageenan . Carrageenan is well known for its gelling property, and it is bioactive because of its sulfate content. The higher the sulphate rate, the more soluble the polymer and the less viscous the solution.
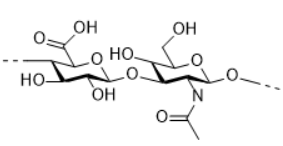
Hyaluronic acid
HA is a natural and unbranched polymer, containing repeating disaccharide units linked by ß-1,4-glycosidic bonds. Each disaccharide consists of N-acetyl-D-glucosamine and D-glucuronic acid connected by ß 1,3-glycosidic bonds. HA is a natural polysaccharide which could be produced by humain body. It plays an important role in cosmetics because of its high wate-retention capacity, anti-aging property, as well as high compatibility with humain body. Today, HA is produced mainly by bioengineering. HA could be crosslinked to increase the stability, which is essential for the aesthetic implant application of HA (Polymers 2018, 10, 701).
Alkyl polyglycoside (APG)
Alkyl polyglycoside is a type of non-ionic surfactant based on alcohol grafted glycoside. The APG is a green surfactant which means bio origined and biodegradable. Most part of APG are synthesized from glucose, but during last few decades, new APG based on algae polysaccharides such as alginate, ulvan were synthesized by green chemistry (Green Chem., 2016,18, 6573-6585; J Appl Phycol 2019, 31, 1931–1946).
Attemps to have new type of APGs such as disaccharide hydrophilic head, double hydrophobic tails were also reported (ChemistrySelect, 2021, 6, 389-395), which could no doubt enlarge the application of APG, but still some distance to go to achieve industrial application.
Guar gum
Guar gum is derived from the seeds of the plant Cyamopsis tetragonoloba. The primary structure of guar is ß-1,4 linked galactose et mannose, with 1,6-linked galactose residues at every second mannose, forming short side-branches. Guar gum was used for thickening the formulation because of the high viscosity of its water solution (J Food Sci Technol, 2014, 51(3): 409–418).
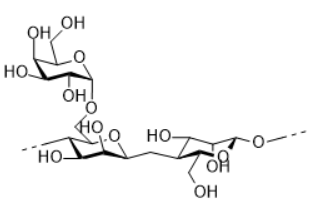
There are other polysaccharides of galactomannan like locustbean gum and tara gum, the difference lies in the ratio of galactose and mannose.
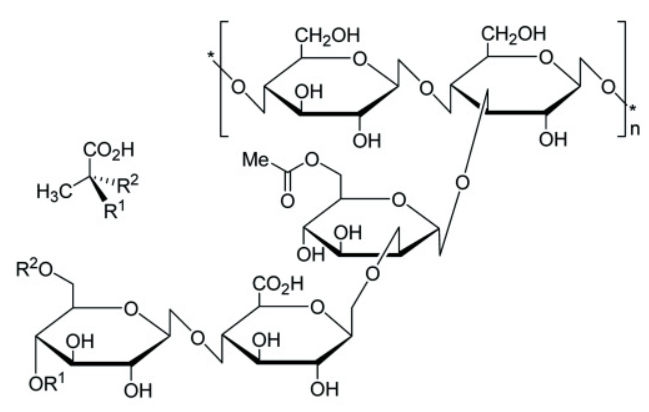
Xanthan gum
XG is a fermentation product of the Gram-negative bacterium Xanthomonas campestris. Chemically, it consists of β-1,4-D-glucopyranose glucan backbone with a pendant trisaccharide side chain, composed of mannose (β-1,4), glucuronic acid (β-1,2) and terminal mannose residues. The side chain is attached to alternate glucose residue in the backbone by α-1,3 linkages. In the side chain, the terminal mannose moiety is partially substituted with a pyruvate residue linked as an acetal to the 4- and 6-positions; the internal mannose sugar closest to the backbone is acetylated at C-6. The O-acetyl and pyruvate residues deprotonate at pH > 4.5, increase charge density along the xanthan chains, thus enabling XG for Ca2+ ion-mediated physical crosslinking. Xanthan gum is an anionic polysaccharide because of the carboxylic acid group on the sidechain, it could be used as thickening agent and stabilizer. Xanthan could be crosslinked physically or chemically with other polysaccharide to form hydrogel. (RSC Adv., 2020,10, 27103-27136)