When characterizing a polymer, several pieces of information must be determined. We have seen in the articles “How to characterize the chemical nature of polymers” and “How to characterize the chain of a polymer“, the techniques used for these characterizations. The knowledge of the thermal properties of solid polymers is important, because it is directly related to the mechanical characteristics of the materials.
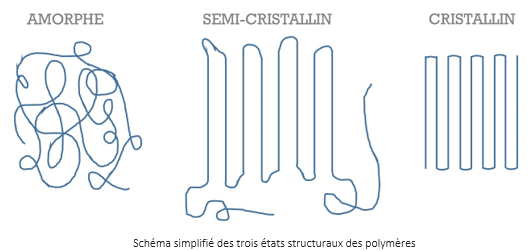
Solid polymers exist in three distinct states:
- The amorphous state: corresponds to the disordered entanglement of polymer chains
- The crystalline state: the arrangement of the polymer chains is ordered, it is concretized by the formation of monocrystals of small dimensions
- The semi-crystalline state : results from the conjugation of the two previous states
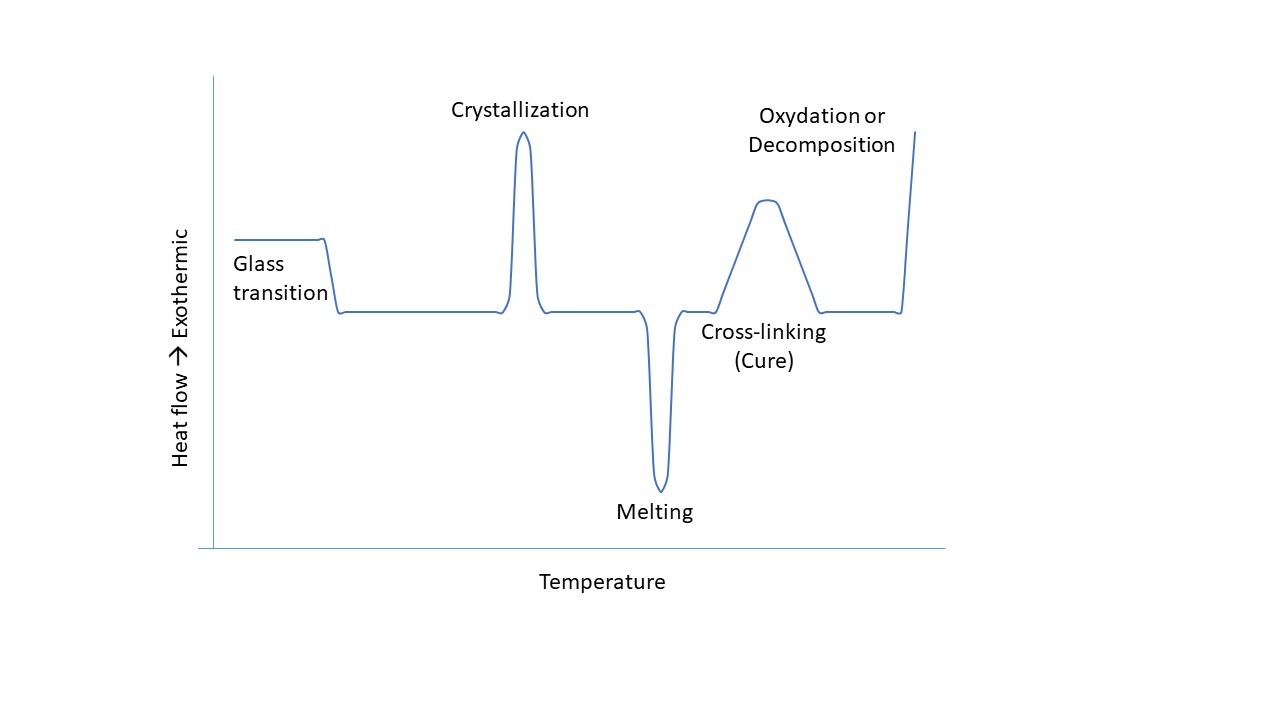
Polymeric materials go through several physical states during a temperature change: rubbery, glassy or viscous liquid. Depending on the use of the material or the selected processing method, it is preferable to know the temperatures at which these phase changes take place. Moreover, the identification of the transition temperatures allows to determine the structural state of the polymer. Indeed, crystalline and semi-crystalline materials have a melting temperature. While, the amorphous and semi-crystalline materials, have, as for them, a glass transition temperature (Tg)
This temperature, specific to polymers, is at the interface between the rubbery and glassy states. It defines the minimum temperature of use of an elastomer as well as the maximum temperature of use of an amorphous polymer in the glassy state. In addition, it indicates the minimum processing temperature of polymeric materials. It can be influenced by the molar mass of the polymer, its molecular structure and the use of plasticizer.Other techniques can also be used to highlight the specific temperatures of polymers. For example, dynamic mechanical analysis (DMA) allows to determine the glass transition temperature, the Young’s modulus (E’), the Coulomb modulus (G’) and the loss factor (delta tangent). If you wish to characterize one of your materials, we invite you to contact our analytical department to help you choose the most appropriate analytical technique.
Differential Scanning Calorimetry, or DSC, is an analytical technique used to study the transition temperatures of polymers. The principle of use of this apparatus is based on the comparison of two samples, one containing the polymer and the other not. The two samples are heated in an oven in a simultaneous and identical way. The device then measures the difference in enthalpy between the two samples. Indeed, the changes of state of the polymer result in endothermic or exothermic reactions that can be characterized by recording the temperature versus time. For example, an endothermic peak will correspond to the melting of a crystalline phase.
Other techniques can also be used to highlight the specific temperatures of polymers. For example, dynamic mechanical analysis (DMA) allows to determine the glass transition temperature, the Young’s modulus (E’), the Coulomb modulus (G’) and the loss factor (delta tangent). If you wish to characterize one of your materials, we invite you to contact our analytical department to help you choose the most appropriate analytical technique.
For more information, please contact us !

